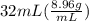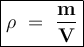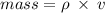Chemistry, 12.07.2019 22:30, annette211pdd8v9
You are asked to determine the mass of a piece of copper using its reported density, 8.96 g/ml, and a 150-ml graduated cylinder. first, you add 105 ml of water to the graduated cylinder; then you place the piece of copper in the cylinder and record a volume of 137 ml. what is the mass of the copper reported with the correct number of significant figures?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
Do you know the correct answer?
You are asked to determine the mass of a piece of copper using its reported density, 8.96 g/ml, and...
Questions in other subjects:

Chemistry, 11.12.2020 04:40

Mathematics, 11.12.2020 04:40


Mathematics, 11.12.2020 04:40

Mathematics, 11.12.2020 04:40

History, 11.12.2020 04:40


Biology, 11.12.2020 04:40

Physics, 11.12.2020 04:40

English, 11.12.2020 04:40