
Let us assume that fe(oh)2(s) is completely insoluble, which signifies that the precipitation reaction with naoh(aq) (presented in the transition) would go to completion. fe2+(aq)+2naoh(aq) → fe(oh)2(s)+2na+(aq) if you had a 0.500 l solution containing 0.0230 m of fe2+(aq), and you wished to add enough 1.29 m naoh(aq) to precipitate all of the metal, what is the minimum amount of the naoh(aq) solution you would need to add? assume that the naoh(aq) solution is the only source of oh−(aq) for the precipitation.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
Do you know the correct answer?
Let us assume that fe(oh)2(s) is completely insoluble, which signifies that the precipitation reacti...
Questions in other subjects:



Mathematics, 01.10.2019 15:30

Social Studies, 01.10.2019 15:30

Mathematics, 01.10.2019 15:30

Chemistry, 01.10.2019 15:30

Chemistry, 01.10.2019 15:30


Computers and Technology, 01.10.2019 15:30



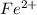 is as follows:-
is as follows:-![Fe^{2+} = 500 mL Fe^{2+} * \frac{0.0230mole\ Fe^{2+}]}{[1 mol\ Fe^{2+}}](/tpl/images/0082/8691/681c2.png) = 11.50 mmol Fe^(2+)
= 11.50 mmol Fe^(2+)![11.50\ mmol\ Fe^{2+} * \frac{[2 \ mol\ NaOH]}{[1 \mol Fe^{2+}}](/tpl/images/0082/8691/9368e.png) = 23.00 mmol NaOH
= 23.00 mmol NaOH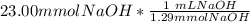 = 17.8 mL NaOH.
= 17.8 mL NaOH.



