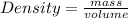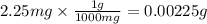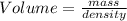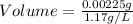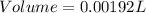Chemistry, 13.07.2019 04:30, fernandezvela27
Agas sample has a density of 1.17 g/l. what volume of this gas will havea mass of 2.25 mg?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2



Chemistry, 23.06.2019 06:30, artbydi
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
Do you know the correct answer?
Agas sample has a density of 1.17 g/l. what volume of this gas will havea mass of 2.25 mg?...
Questions in other subjects:



Mathematics, 21.12.2020 22:00

Mathematics, 21.12.2020 22:00



Mathematics, 21.12.2020 22:00



Mathematics, 21.12.2020 22:00