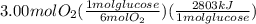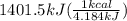Chemistry, 13.07.2019 08:00, zekrader18
The combustion of glucose (c6h12o6) with oxygen gas produces carbon dioxide and water. this process releases 2803 kj per mole of glucose. when 3.00 mol of oxygen react in this way with glucose, what is the energy release in kcal? (hint: write a balanced equation for the combustion process.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, kawaiiblurainbow
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 22:10, zwbaby3693
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
Do you know the correct answer?
The combustion of glucose (c6h12o6) with oxygen gas produces carbon dioxide and water. this process...
Questions in other subjects:

Mathematics, 07.04.2021 05:30


History, 07.04.2021 05:30


Business, 07.04.2021 05:30


Mathematics, 07.04.2021 05:30

Mathematics, 07.04.2021 05:30


Mathematics, 07.04.2021 05:30