
Chemistry, 13.07.2019 11:30, heggestade
A100 gram glass container contains 200 grams of water and 50.0 grams of ice all at 0°c. a 200 gram piece of lead at 100°c is added to the water and ice in the container. what is the final temperature of the system? (specific heat of ice = 2,000 j/kg°c , specific heat of water = 4,186 j/kg°c, heat of fusion of water = 333.7 kj/kg, specific heat of glass = 837.2 j/km°c, specific heat of lead = 127.7 j/km°c)

Answers: 1
Similar questions

Chemistry, 25.06.2019 23:30, tia5520
Answers: 1

Chemistry, 10.07.2019 07:00, Spoilmom1901
Answers: 1

Business, 08.10.2019 21:00, helpmewithmath70
Answers: 3

Chemistry, 16.11.2019 06:31, xxaurorabluexx
Answers: 1
Do you know the correct answer?
A100 gram glass container contains 200 grams of water and 50.0 grams of ice all at 0°c. a 200 gram p...
Questions in other subjects:


Mathematics, 24.03.2021 08:20

French, 24.03.2021 08:20

Mathematics, 24.03.2021 08:20


Mathematics, 24.03.2021 08:30

Biology, 24.03.2021 08:30

Mathematics, 24.03.2021 08:30



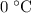
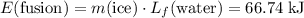 and
and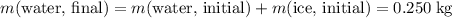
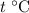 . Thus
. Thus 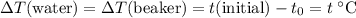
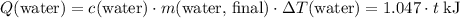 (converted to kilojoules)
(converted to kilojoules) 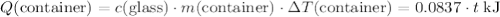
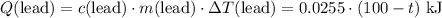
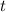 .
. 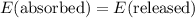
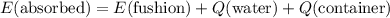
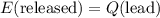
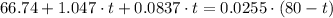
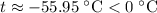 which goes against the initial assumption. Implying that the final temperature does not go above the melting point of water- i.e.,
which goes against the initial assumption. Implying that the final temperature does not go above the melting point of water- i.e., 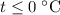 . However, there's no way for the temperature of the system to go below
. However, there's no way for the temperature of the system to go below 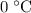 ; doing so would require the removal of heat from the system which isn't possible under the given circumstance; the ice-water mixture experiences an addition of heat as the hot block of lead was added to the system.
; doing so would require the removal of heat from the system which isn't possible under the given circumstance; the ice-water mixture experiences an addition of heat as the hot block of lead was added to the system.



