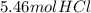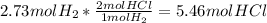Chemistry, 13.07.2019 11:30, auviannadority13
For the reaction, calculate how many moles of the product form when 2.73 mol of h2 completely reacts. assume that there is more than enough of the other reactant. h2(g)+cl2(g)→2hcl(g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, notkeandre9
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1

Do you know the correct answer?
For the reaction, calculate how many moles of the product form when 2.73 mol of h2 completely reacts...
Questions in other subjects:



Mathematics, 23.11.2021 23:40


Geography, 23.11.2021 23:40


Social Studies, 23.11.2021 23:40

English, 23.11.2021 23:40

Mathematics, 23.11.2021 23:40

Biology, 23.11.2021 23:40