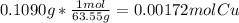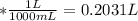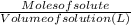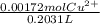Chemistry, 13.07.2019 20:00, masonorourke
A0.1090 g sample of copper metal is dissolved in 60. ml of concentrated hno3 to form cu2+ ions and then water is added to make a total volume of 203.1 ml. (calculate the molarity of cu2+.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Do you know the correct answer?
A0.1090 g sample of copper metal is dissolved in 60. ml of concentrated hno3 to form cu2+ ions and t...
Questions in other subjects:



Chemistry, 06.05.2021 18:30

Engineering, 06.05.2021 18:30

Biology, 06.05.2021 18:30

English, 06.05.2021 18:30




Chemistry, 06.05.2021 18:30