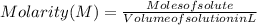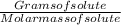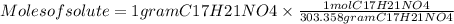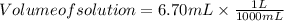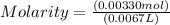Chemistry, 13.07.2019 20:00, myrkaxsanchezz
The free-base form of cocaine has a solubility of 1.00 g in 6.70 ml ethanol (ch3ch2oh). calculate the molarity of a saturated solution of the free-base form of cocaine in ethanol.

Answers: 1
Similar questions

Chemistry, 15.07.2019 18:40, pierreangie17
Answers: 3

Chemistry, 27.07.2019 10:30, lcaulkett27
Answers: 1
Do you know the correct answer?
The free-base form of cocaine has a solubility of 1.00 g in 6.70 ml ethanol (ch3ch2oh). calculate th...
Questions in other subjects:



Business, 03.08.2019 11:30



Mathematics, 03.08.2019 11:30




Mathematics, 03.08.2019 11:30