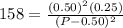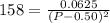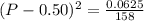Chemistry, 13.07.2019 22:00, elijahdouglass00
Asample of pure no2 gas decomposes at 1000 k 2no2 (g) ↔ 2 no (g) + o2 (g) the constant kp is 158. an analysis shows that the partial pressure of o2 is 0.25 atmospheres at equilibrium. determine the pressure of no and no2.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 02:00, bernicewhite156
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
Do you know the correct answer?
Asample of pure no2 gas decomposes at 1000 k ...
Questions in other subjects:









Mathematics, 08.04.2021 18:40

Advanced Placement (AP), 08.04.2021 18:40


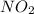 = 0.02 atm.
= 0.02 atm.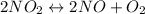
![Kp=(\frac{[NO]^2[O_2]}{[NO_2]^2})](/tpl/images/0086/2744/569f6.png)