
Chemistry, 13.07.2019 22:30, karrathomas
The osmotic pressure of a solution formed by dissolving 35.0 mg of aspirin (c9h8o4) in 0.250 l of water at 25°c is atm.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1


Chemistry, 23.06.2019 12:30, asseatingbandit
You have 125 g of a certain seasoning and are told that it contains 70.0 g of salt. what is the peroentage of salt by mass in this seasoning?
Answers: 2
Do you know the correct answer?
The osmotic pressure of a solution formed by dissolving 35.0 mg of aspirin (c9h8o4) in 0.250 l of wa...
Questions in other subjects:

Biology, 10.03.2022 23:00

English, 10.03.2022 23:00









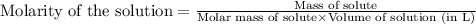

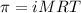
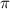 = osmotic pressure of the solution
= osmotic pressure of the solution 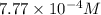
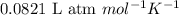
![25^oC=[273+25]=298K](/tpl/images/0086/3926/6a9f9.png)






