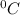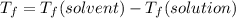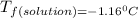Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1


Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
Do you know the correct answer?
Calculate the freezing point of a solution made from 22.0 g of octane (c8h18) dissolved in 148.0 g o...
Questions in other subjects:


Mathematics, 07.12.2020 20:40

Biology, 07.12.2020 20:40

Engineering, 07.12.2020 20:40

English, 07.12.2020 20:40

English, 07.12.2020 20:40

English, 07.12.2020 20:40


Biology, 07.12.2020 20:40

Mathematics, 07.12.2020 20:40


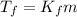
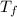 is the change in the freezing point of the solvent.
is the change in the freezing point of the solvent.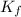 of benzene is 5.12
of benzene is 5.12
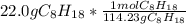 = 0.193 mol
= 0.193 mol

 ) (0.130 m) " alt=" 5.12^{0}C/m" />) (0.130 m) " />
) (0.130 m) " alt=" 5.12^{0}C/m" />) (0.130 m) " />