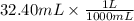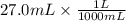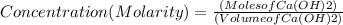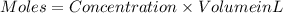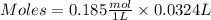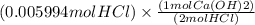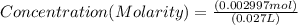Chemistry, 14.07.2019 02:30, fespinoza019
If 27.0 ml of ca(oh)2 with an unknown concentration is neutralized by 32.40 ml of 0.185 m hcl, what is the concentration of the ca(oh)2 solution? show all of the work needed to solve this problem. (2 points) ca(oh)2 + 2hcl yields 2h2 o + cacl2

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
Do you know the correct answer?
If 27.0 ml of ca(oh)2 with an unknown concentration is neutralized by 32.40 ml of 0.185 m hcl, what...
Questions in other subjects:



History, 15.01.2021 22:40

Mathematics, 15.01.2021 22:40

Chemistry, 15.01.2021 22:40


Mathematics, 15.01.2021 22:40

Mathematics, 15.01.2021 22:40