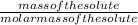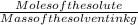Chemistry, 14.07.2019 04:00, alexcute965
Asolution is made by dissolving 21.5 grams of glucose (c6h12o6) in 255 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Do you know the correct answer?
Asolution is made by dissolving 21.5 grams of glucose (c6h12o6) in 255 grams of water. what is the f...
Questions in other subjects:






Mathematics, 02.12.2021 01:00



Spanish, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00