
Chemistry, 14.07.2019 06:00, achoward08
In the hypothetical reaction below, substance a is consumed at a rate of 2.0 mol/l·s. if this reaction is at dynamic equilibrium, at what rate will substance b be consumed? 0.0 mol/l·s 1.0 mol/l·s 2.0 mol/l·s 4.0 mol/l·s

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, YatesDevon3371
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1


Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 02:00, Robloxdemonduckyt
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
Do you know the correct answer?
In the hypothetical reaction below, substance a is consumed at a rate of 2.0 mol/l·s. if this reacti...
Questions in other subjects:



History, 27.04.2021 03:30


Mathematics, 27.04.2021 03:30

Mathematics, 27.04.2021 03:30


Social Studies, 27.04.2021 03:30


Chemistry, 27.04.2021 03:30


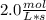
![R(a) = k * \frac{d[A]}{dt}](/tpl/images/0087/5945/2a16d.png)
![R (b)= k * \frac{d[B]}{dt}](/tpl/images/0087/5945/bbb74.png)




