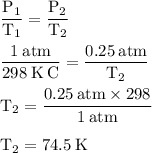
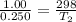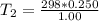Arigid cylinder contains 2.00 liters of gas at a temperature of 25°c. if the pressure of this gas is changed from 1.00 atmospheres to 0.250 atmospheres, what will be the new temperature (in kelvin, reported to three significant figures) of the gas? (the volume is constant.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
Do you know the correct answer?
Arigid cylinder contains 2.00 liters of gas at a temperature of 25°c. if the pressure of this gas is...
Questions in other subjects:



Physics, 28.10.2020 17:40

Mathematics, 28.10.2020 17:40

Biology, 28.10.2020 17:40



Mathematics, 28.10.2020 17:40



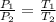
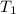 =25+273=298K
=25+273=298K
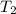 = ?
= ?
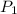 = 1.00 atm
= 1.00 atm
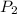 = 0.250 atm
= 0.250 atm