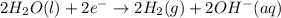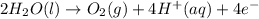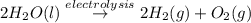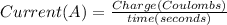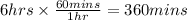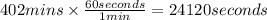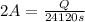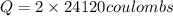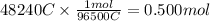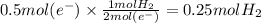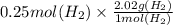Chemistry, 14.07.2019 10:00, nekathadon
In the electrolysis of water shown below, a current of 2 amps is applied to 180 ml of h2o(l) for 6 hours and 42 minutes. how many grams of h2(g) are formed? (faraday's constant = 96,500 c/mol)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, ian2006huang
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3


Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
Do you know the correct answer?
In the electrolysis of water shown below, a current of 2 amps is applied to 180 ml of h2o(l) for 6 h...
Questions in other subjects:




Mathematics, 07.10.2021 01:00

Physics, 07.10.2021 01:00



Mathematics, 07.10.2021 01:00

Physics, 07.10.2021 01:00

English, 07.10.2021 01:00