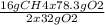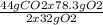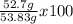Consider the following reaction. ch4 (g) + o2 (g) co2 (g) + h2o (l) a chemist allows 23.2g of ch4 and 78.3g o2 to react. when the reaction is finished, the chemist collects 52.7g co2. determine the limiting reagent, theoretical yield, and percent yield for the reaction

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1
Do you know the correct answer?
Consider the following reaction. ch4 (g) + o2 (g) co2 (g) + h2o (l) a chemist allows 23.2g of ch4 an...
Questions in other subjects:

SAT, 27.01.2022 01:00



English, 27.01.2022 01:00






Mathematics, 27.01.2022 01:00