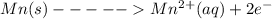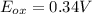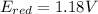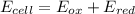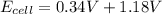Chemistry, 14.07.2019 14:30, sciencecreation87
An electrochemical cell has the following overall reaction: mn(s) + cu2+(aq) > cu(s) + mn2+(aq) the reduction half-reaction has a standard potential of 1.18 v. the oxidation half-reaction has a standard potential of 0.34 v. what is the overall cell potential? (a) -1.52 v (b) -1.18 v (c) +1.18 v (d) +1.52 v

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, markmlg122
Two things that biomedical has invented or innvated
Answers: 1

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
Do you know the correct answer?
An electrochemical cell has the following overall reaction: mn(s) + cu2+(aq) > cu(s) + mn2+(aq)...
Questions in other subjects:




Spanish, 09.05.2020 02:57

Spanish, 09.05.2020 02:57


Chemistry, 09.05.2020 02:57



Mathematics, 09.05.2020 02:57