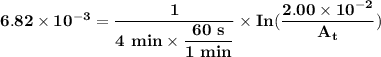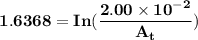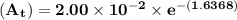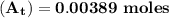Chemistry, 14.07.2019 17:30, guccikathyyy6195
The first-order rate constant for the decomposition of n2o5, 2n2o5(g)→4no2(g)+o2(g) at 70∘c is 6.82×10−3 s−1. suppose we start with 2.00×10−2 mol of n2o5(g) in a volume of 2.3 l . you may want to reference (page) section 14.4 while completing this problem. part a how many moles of n2o5 will remain after 4.0 min ? ,

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
Do you know the correct answer?
The first-order rate constant for the decomposition of n2o5, 2n2o5(g)→4no2(g)+o2(g) at 70∘c is 6.82×...
Questions in other subjects:

Mathematics, 09.06.2021 17:50

Mathematics, 09.06.2021 17:50


Mathematics, 09.06.2021 17:50

History, 09.06.2021 17:50


Mathematics, 09.06.2021 17:50

Biology, 09.06.2021 17:50

Biology, 09.06.2021 17:50


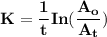
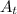 = ???
= ???