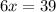Chemistry, 14.07.2019 22:30, maryalice2002
When 6.0 mol al react with 13 mol hcl, what is the limiting reactant, and how many moles of h2 can be formed? 2 al + 6hcl → 2 alcl3 + 3 h2 select one: a. al is the limiting reactant, 9.0 mol h2 can be formed b. hcl is the limiting reactant, 6.5 mol h2 can be formed c. al is the limiting reactant, 6.0 mol h2 can be formed d. hcl is the limiting reactant, 4.3 mol h2 can be formed

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
Do you know the correct answer?
When 6.0 mol al react with 13 mol hcl, what is the limiting reactant, and how many moles of h2 can b...
Questions in other subjects:



Social Studies, 03.12.2020 18:40



Biology, 03.12.2020 18:40


Biology, 03.12.2020 18:40

Biology, 03.12.2020 18:40

Mathematics, 03.12.2020 18:40


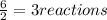

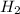 that are produced with 13 moles of HCl.
that are produced with 13 moles of HCl.