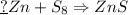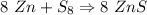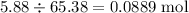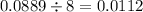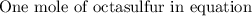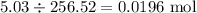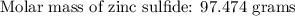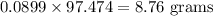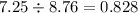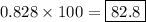Chemistry, 15.07.2019 05:00, xxaurorabluexx
If 5.88 grams of zn react with 5.03 grams of s8 to produce 7.25 grams of zns, what are the theoretical yield and percent yield of this reaction? be sure to show the work that you did to solve this problem. unbalanced equation: zn + s8 yields zns

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, froyg1234
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
Do you know the correct answer?
If 5.88 grams of zn react with 5.03 grams of s8 to produce 7.25 grams of zns, what are the theoretic...
Questions in other subjects:



Health, 18.10.2019 22:10


Mathematics, 18.10.2019 22:10



English, 18.10.2019 22:10

Computers and Technology, 18.10.2019 22:10

History, 18.10.2019 22:10