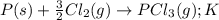Chemistry, 15.07.2019 13:00, pablogonzaleztellez
Consider the reaction: p(s) + 3/2 cl2( g. pcl3( g. write the equilibrium constant for this reaction in terms of the equilibrium constants, ka and kb, for reactions a and b below: a.)p(s) + 5/2 cl2( g. pcl5( g. ka b.)pcl3( g. + cl2( g. pcl5( g. kb

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
Do you know the correct answer?
Consider the reaction: p(s) + 3/2 cl2( g. pcl3( g. write the equilibrium constant for this reaction...
Questions in other subjects:

History, 22.04.2020 21:48



Social Studies, 22.04.2020 21:48

Mathematics, 22.04.2020 21:49






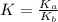
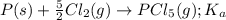
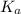 for the above equation is:
for the above equation is:![K_a=\frac{[PCl_5]}{[P][Cl_2]^{5/2}}](/tpl/images/0092/5571/bd7b7.png) ......(1)
......(1)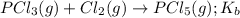
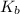 for the above equation is:
for the above equation is:![K_b=\frac{[PCl_5]}{[PCl_3][Cl_2]}](/tpl/images/0092/5571/bd7b0.png) ......(2)
......(2)![\frac{K_a}{K_b}=\left(\frac{\frac{[PCl_5]}{[P][Cl_2]^{5/2}}}{\frac{[PCl_5]}{[PCl_3][Cl_2]}}\right)\\\\\\\frac{K_a}{K_b}=\frac{[PCl_3]}{[P][Cl_2]^{3/2}}](/tpl/images/0092/5571/fcd9a.png)