

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1


Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
Do you know the correct answer?
First: calculate the number of moles of kool-aid powder needed to make 100ml of a 0.1m solution. 0....
Questions in other subjects:










History, 21.06.2019 21:30


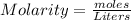
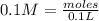
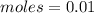
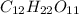 .
.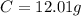
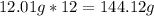
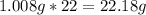
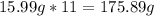
 -> So we know that the molar weight of the Kool-Aid powder is 342.19g.
-> So we know that the molar weight of the Kool-Aid powder is 342.19g.




