
Chemistry, 15.07.2019 17:00, ineedhelp2285
Calculate the pressure of o2 (in atm) over a sample of nio at 25.00°c if δg o = 212 kj/mol for the reaction. for this calculation, use the value r = 8.3144 j/k·mol. nio(s) ⇌ ni(s) + 1 2 o2(g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Do you know the correct answer?
Calculate the pressure of o2 (in atm) over a sample of nio at 25.00°c if δg o = 212 kj/mol for the r...
Questions in other subjects:


Arts, 10.12.2019 13:31

Mathematics, 10.12.2019 13:31

Mathematics, 10.12.2019 13:31

Spanish, 10.12.2019 13:31



Advanced Placement (AP), 10.12.2019 13:31

History, 10.12.2019 13:31

Mathematics, 10.12.2019 13:31


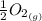 We can find out the pressure on oxygen by using the equation of gibb's free energy with remainder quotient which is, ΔG = ΔG° +RT lnQ
We can find out the pressure on oxygen by using the equation of gibb's free energy with remainder quotient which is, ΔG = ΔG° +RT lnQ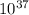
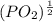 So, (1.51 X
So, (1.51 X  = 3.87 X
= 3.87 X 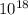
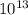 atm
atm



