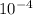Chemistry, 15.07.2019 19:00, lerasteidl
The equilibrium constant, keq, is 4.4 × 10-4 at 500 k. 2nocl (g) < --> 2no(g) + cl2 (g) determine the direction of reaction if 1.00 m nocl is combined with 0.500 m no and 0.500 m cl2 in a sealed flask. a.)no reaction occurs. b.)reaction proceeds to the right toward the products. c.)reaction proceeds to the left toward the reactants. d.)reaction proceeds to the left toward the products.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
Do you know the correct answer?
The equilibrium constant, keq, is 4.4 × 10-4 at 500 k. 2nocl (g) < --> 2no(g) + cl2 (g) deter...
Questions in other subjects:




Spanish, 01.10.2019 09:00





Mathematics, 01.10.2019 09:00


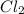
![Q = \frac{[ NO]^{2}. [Cl_{2} ]}{[ NOCl ]^{2}}](/tpl/images/1058/5527/aa67c.png)