
Ethyne (c2 h2 (g), hf = 226.77 kj/mol) undergoes complete combustion in the presence of oxygen to produce carbon dioxide (co2 (g), hf = –393.5 kj/mol ) and water (h2 o(g), hf = –241.82 kj/mol) according to the equation below.what is the enthalpy of combustion (per mole) of c2 h2 (g)? use 

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, Brooke7644
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 04:00, kichensides
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Do you know the correct answer?
Ethyne (c2 h2 (g), hf = 226.77 kj/mol) undergoes complete combustion in the...
Questions in other subjects:

Mathematics, 26.10.2020 05:10

Mathematics, 26.10.2020 05:10






Mathematics, 26.10.2020 05:10


Mathematics, 26.10.2020 05:10


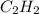 is -1255.6 KJ/mol
is -1255.6 KJ/mol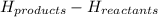
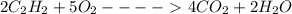
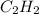 which is -2511.2 KJ/mol
which is -2511.2 KJ/mol



