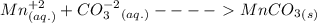Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, froyg1234
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 21.06.2019 22:00, juansantos7b
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 03:30, isabelvaldez123
The boiling point of liquids is very high what does it indicate
Answers: 1
Do you know the correct answer?
Write the net ionic equation for the precipitation of manganese(ii) carbonate from aqueous solution:...
Questions in other subjects:


English, 24.06.2019 06:00


Physics, 24.06.2019 06:00

History, 24.06.2019 06:00


Spanish, 24.06.2019 06:00



English, 24.06.2019 06:00