
Chemistry, 16.07.2019 04:00, preciosakassidy
Asolution of an unknown nonvolatile nonelectrolyte was prepared by dissolving 0.250 g of the substance in 40.0 g of ccl4. the boiling point of the resultant solution was 0.357oc higher than that of the pure solvent. calculate the molar mass of the solute. (kb = 5.02oc/m)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1


Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
Do you know the correct answer?
Asolution of an unknown nonvolatile nonelectrolyte was prepared by dissolving 0.250 g of the substan...
Questions in other subjects:



Mathematics, 21.04.2021 05:10


Mathematics, 21.04.2021 05:10


Mathematics, 21.04.2021 05:10

Mathematics, 21.04.2021 05:10

Social Studies, 21.04.2021 05:10


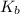 m
m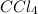 = 40,
= 40,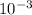 .
. 



