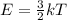Chemistry, 17.07.2019 04:30, thegreentnt5025
You are experimenting on the effect of temperature on the rate of reaction between hydrochloric acid (hcl) and potassium iodide (ki). when the reaction is completed at 273k there are approximately 250,000 collisions per mole of reactant. you run the experiment again at 350k. which of the following would you expect to be the number of collisions recorded at 350k a. 2000 b. 700,000 c. 0 d. 200,000

Answers: 1
Similar questions

Chemistry, 29.06.2019 12:40, rozalee14
Answers: 1

Chemistry, 14.07.2019 19:00, lpssprinklezlps
Answers: 1

Chemistry, 02.09.2019 17:00, babygirlmiller
Answers: 1

Physics, 09.11.2019 23:31, JosefineRubino2204
Answers: 1
Do you know the correct answer?
You are experimenting on the effect of temperature on the rate of reaction between hydrochloric acid...
Questions in other subjects:

Mathematics, 07.09.2021 09:10

Mathematics, 07.09.2021 09:10

Biology, 07.09.2021 09:10

English, 07.09.2021 09:10

Mathematics, 07.09.2021 09:10


Mathematics, 07.09.2021 09:10

English, 07.09.2021 09:10