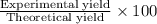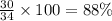Chemistry, 17.07.2019 13:00, ringo12384
When nh3 is prepared from 28 g n2 and excess h2, the theoretical yield of nh3 is 34 g. when this reaction is carried out in a given experiment, only 30. g is produced. what is the percentage yield?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, ayahabdulhaqq2
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 21.06.2019 16:50, nnaomii
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
Do you know the correct answer?
When nh3 is prepared from 28 g n2 and excess h2, the theoretical yield of nh3 is 34 g. when this rea...
Questions in other subjects:

English, 10.11.2020 01:00

History, 10.11.2020 01:00


Mathematics, 10.11.2020 01:00

Computers and Technology, 10.11.2020 01:00

Biology, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00


Biology, 10.11.2020 01:00