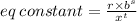Chemistry, 18.07.2019 03:00, baptistatm51976
In the reversible reaction shown below, r moles of a react with s moles of b to produce t moles of c. which equation can be used to represent the equilibrium constant for the forward reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, ayoismeisjjjjuan
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x. xx ml
Answers: 1

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Do you know the correct answer?
In the reversible reaction shown below, r moles of a react with s moles of b to produce t moles of c...
Questions in other subjects:


Biology, 10.11.2019 05:31

Computers and Technology, 10.11.2019 05:31

History, 10.11.2019 05:31

History, 10.11.2019 05:31

Mathematics, 10.11.2019 05:31

Mathematics, 10.11.2019 05:31