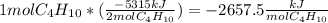Butane (c4 h10(g), mc031-1.jpghf = –125.6 kj/mol) reacts with oxygen to produce carbon dioxide (co2 , mc031-2.jpghf = –393.5 kj/mol ) and water (h2 o, mc031-3.jpghf = –241.82 kj/mol) according to the equation below. mc031-4.jpg what is the enthalpy of combustion (per mole) of c4h10 (g)? use mc031-5.jpg. –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2



Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
Do you know the correct answer?
Butane (c4 h10(g), mc031-1.jpghf = –125.6 kj/mol) reacts with oxygen to produce carbon dioxide (co2...
Questions in other subjects:

Computers and Technology, 10.10.2021 07:20

Mathematics, 10.10.2021 07:20

Mathematics, 10.10.2021 07:20


Mathematics, 10.10.2021 07:20


Spanish, 10.10.2021 07:20


Mathematics, 10.10.2021 07:20


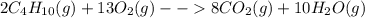
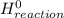 = Σ
= Σ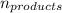 Δ
Δ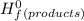 -Σ
-Σ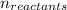 Δ
Δ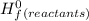
![[{8*(-393.5kJ/mol)}+{10*(-241.82kJ/mol)}]-[{2*(-125.6kJ/mol)}+13*(0 kJ/mol)}]](/tpl/images/0106/1493/0d4a2.png) =[-3148kJ/mol+(-2418.2kJ/mol)]-[(-251.2kJ/mol)+0]
=[-3148kJ/mol+(-2418.2kJ/mol)]-[(-251.2kJ/mol)+0]