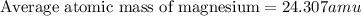Chemistry, 19.07.2019 19:00, erikagibson3414
The naturally occurring isotopes of magnesium are magnesium-24. magnesium-25, and magnesium-26. magnesium-24 has an abundance of 78.994% and a mass of 23.985 amu. magnesium-25 has an abundance of 10.001% and a mass of 24.986 amu. magnesium-26 has an abundance of 11.013% and a mass of 25.983 amu. calculate the average atomic mass of magnesium.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
Do you know the correct answer?
The naturally occurring isotopes of magnesium are magnesium-24. magnesium-25, and magnesium-26. magn...
Questions in other subjects:


Mathematics, 03.06.2021 18:10


Mathematics, 03.06.2021 18:10



Mathematics, 03.06.2021 18:10

SAT, 03.06.2021 18:10



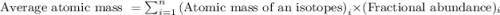 .....(1)
.....(1)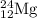 isotope:
isotope: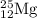 isotope:
isotope: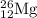 isotope:
isotope:![\text{Average atomic mass of magnesium}=[(23.985\times 0.78994)+(24.986\times 0.10001)+(25.983\times 0.11013)]](/tpl/images/0108/8899/cfd57.png)