

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, kaliloabousjbf
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1
Do you know the correct answer?
Cl2(g) + 2nabr(aq) 2nacl(aq) + br2(l) the reaction given above is a single replacement reaction beca...
Questions in other subjects:


Advanced Placement (AP), 19.09.2021 19:10


Mathematics, 19.09.2021 19:10




Mathematics, 19.09.2021 19:10



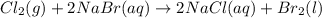
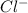 in
in 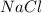 and
and 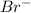 in
in  donates electrons to form
donates electrons to form  .
.




