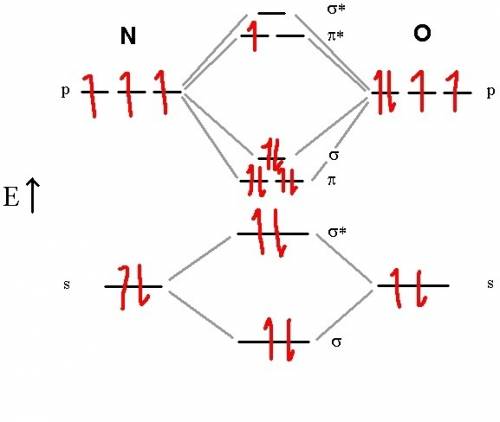
Chemistry, 20.07.2019 04:30, ramondoss249
According to the molecular orbital (mo) treatment of the no molecule, what are the bond order and the number of unpaired electrons, respectively?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3
Do you know the correct answer?
According to the molecular orbital (mo) treatment of the no molecule, what are the bond order and th...
Questions in other subjects:



Physics, 03.01.2020 13:31

Mathematics, 03.01.2020 13:31

Mathematics, 03.01.2020 13:31

Chemistry, 03.01.2020 13:31

Mathematics, 03.01.2020 13:31

Mathematics, 03.01.2020 13:31

Physics, 03.01.2020 13:31


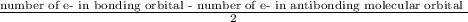
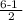 = 2.5
= 2.5