
Chemistry, 24.09.2019 17:40, annamcda301
Check all correct statements concerning pentyne.
contains 3 carbons
contains 5 carbons
contains 7 carbons
has only single bonds
has a double bond
has a triple bond
is an alkyne
is an alkene
is an alkane
which statement is not true of alkenes?
they are unsaturated.
they are reactive.
they have double bonds.
they are more stable than alkanes.
butene would have 4 carbon atoms and a bond.
single
triple
double
which type of reaction to alkenes generally undergo?
substitution
displacement
redox
addition
if the carbon atom of hydrocarbon is bonded to less than 4 other atoms, the hydrocarbon is considered
aromatic
diluted
saturated
unsaturated
which of the following apply to unsaturated hydrocarbons? select all that apply.
they do not have isomers.
they are more stable than saturated hydrocarbons.
they will react readily with other elements and compounds.
they have double and triple bonds.
they only have single bonds.
a straight chain hydrocarbon with the formula c5h10:
has a triple c-c bond
has a double c-c bond
is essentially inert
is unstable and extremely reactive
what is the general formula for alkenes?
cnh2n+2
cnh2n
cnh2n+1
ch2n+2
choose the statements about benzene that are correct.
all benzene hydrocarbons are cnhn+2
all c-c bonds share 2 pairs of electrons.
three c-c bonds share 2 pairs of electrons.
all of the c-c bonds are actually identical.
the structure of the molecule is closed like a ring structure.
the last one is attached as a pic.
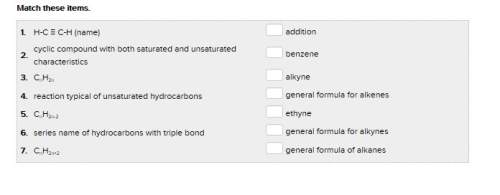

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 23.06.2019 02:00, hannabeth91
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
Do you know the correct answer?
Check all correct statements concerning pentyne.
contains 3 carbons
contains 5 carbons<...
contains 3 carbons
contains 5 carbons<...
Questions in other subjects:

English, 03.04.2021 16:20




Chemistry, 03.04.2021 16:20


Mathematics, 03.04.2021 16:20








