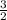Chemistry, 20.07.2019 23:30, jeffreyaxtell4542
One way of obtaining pure sodium carbonate is through the decomposition of the mineral trona, na3(co3)(hco3)·2h2o. 2na3(co3)(hco3)·2h2o(s) → 3na2co3(s) + co2( g. + 5h2o( g. when 1.00 metric ton (1.00 × 103 kg) of trona is decomposed, 0.650 metric ton of na2co3 is recovered. what is the percent yield of this reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 23:00, chastineondre7979
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
Do you know the correct answer?
One way of obtaining pure sodium carbonate is through the decomposition of the mineral trona, na3(co...
Questions in other subjects:

Computers and Technology, 03.10.2021 16:00


English, 03.10.2021 16:00

Computers and Technology, 03.10.2021 16:00

Mathematics, 03.10.2021 16:00

Mathematics, 03.10.2021 16:00


English, 03.10.2021 16:00

Mathematics, 03.10.2021 16:00

Mathematics, 03.10.2021 16:00