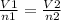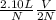Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 23.06.2019 07:00, phancharamachasm
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1

Chemistry, 23.06.2019 16:30, aloading2256
Answer immediately when two or more atoms of the same element are in a chemical bond, the substance is called a(n) a. compound b. molecule c. nucleus
Answers: 1
Do you know the correct answer?
If the number of moles of a gas initially contained in a 2.10 l vessel is doubled, what is the final...
Questions in other subjects:

Mathematics, 05.05.2020 12:13

History, 05.05.2020 12:13






Mathematics, 05.05.2020 12:13