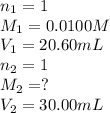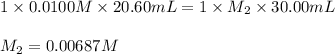Chemistry, 21.07.2019 03:31, arlettehl1011
If 20.60 ml of 0.0100 m aqueous hcl is required to titrate 30.00 ml of an aqueous solution of naoh to the equivalence point, what is the molarity of the naoh solution?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
Do you know the correct answer?
If 20.60 ml of 0.0100 m aqueous hcl is required to titrate 30.00 ml of an aqueous solution of naoh t...
Questions in other subjects:

Mathematics, 04.02.2020 06:52



Mathematics, 04.02.2020 06:52


Mathematics, 04.02.2020 06:52

Health, 04.02.2020 06:52


Mathematics, 04.02.2020 06:52



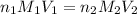
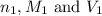 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 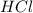
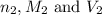 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.