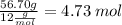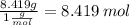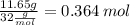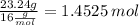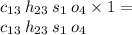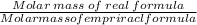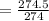Would you please help need asap
The analysis of a compound gives the following percent composition by mass:
C: 56.70 percent; H: 8.419 percent; S: 11.65 percent; O: 23.24 percent. What is its molecular formula, given that its molar mass is 275.4 g?
C?H?S?O?
C atoms
H atoms
S atoms
O atoms

Answers: 1
Other questions on the subject: Biology


Biology, 21.06.2019 23:30, brittanysanders
Melting glaciers is a serious threat to the environment what is the possible consequence of melting glaciers on polar bears?
Answers: 2

Biology, 22.06.2019 02:00, vlactawhalm29
How is the national wildlife refuge system similar to the pacific region coastal program? a. both programs are concerned with providing habitats for wildlife b. both programs are primarily concerned with preserving fish species c. both programs have set aside 150 million acres of land d. both programs are under the u. s. fish and wildlife service
Answers: 3

Biology, 22.06.2019 10:00, tuetheturtle
The double bond between a carbon atom and two oxygen atoms (a molecule of carbon dioxide) has two characteristics. what are they? a. an ionic bond is formed between the oxygen and carbon atoms. b. four valence electrons are shared. c. two valence electrons are shared. d. valence electrons are shared between oxygen atoms.
Answers: 1
Do you know the correct answer?
Would you please help need asap
The analysis of a compound gives the following percent composition...
Questions in other subjects:



Mathematics, 09.01.2020 06:31




Mathematics, 09.01.2020 06:31