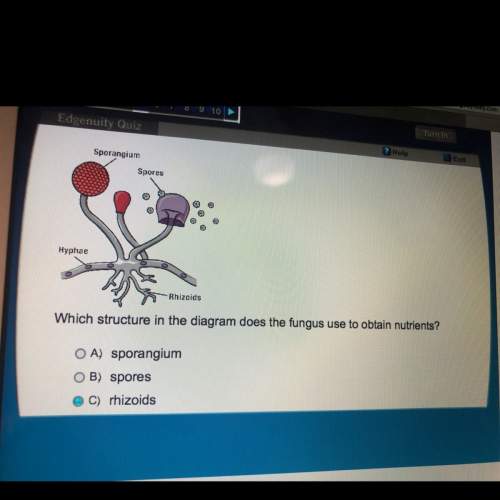
Biology, 09.03.2021 04:00, aloading6574
The pH is a measure of the concentration of the hydrogen ion (H+) in water. Highly acidic water has a very high H+ concentration, while basic water has very low H+ concentrations. The pH scale is a logarithmic scale, such that a difference of one pH unit means a 10-fold difference in H+ concentration. The mathematical relationship between pH and H+ concentration ([H+]) is
pH = –log [H+] OR [H+] = 10-pH where [H+] is in moles per liter.
Use a scientific calculator to calculate [H+] for the following pH values:
7 (a neutral solution)
5.6 (unpolluted rainwater)
3.7 (first acid rain sample in North America)
How many times higher is the concentration of H+ in the Hubbard Brook sample than in unpolluted rainwater?

Answers: 1
Other questions on the subject: Biology


Biology, 22.06.2019 15:30, camiee13dvivvj
Which environmental change would most likely result in bullfrog offspring that are able to store more water than bullfrogs in previous generations?
Answers: 1
Do you know the correct answer?
The pH is a measure of the concentration of the hydrogen ion (H+) in water. Highly acidic water has...
Questions in other subjects:


Biology, 24.10.2020 06:50

Mathematics, 24.10.2020 06:50


History, 24.10.2020 06:50

English, 24.10.2020 06:50


Mathematics, 24.10.2020 06:50