Help please!
(Ignore the chosen answer, I just picked random.)
...

Biology, 20.01.2021 16:00, adrian128383
Help please!
(Ignore the chosen answer, I just picked random.)
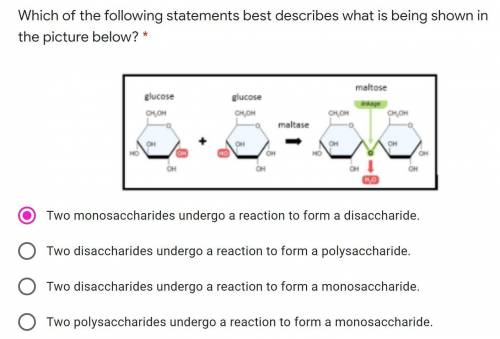

Answers: 1
Other questions on the subject: Biology



Biology, 22.06.2019 08:40, keilyjaramillo2870
What best explains whether bromine (br) or neon (ne) is more likely to form a covalent bond? bromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfills the octet rule. bromine forms covalent bonds because it has many electron shells, but neon has only two electron shells and is tightly bound to its electrons. neon forms covalent bonds because it can share its valence electrons, but bromine has seven valence electrons and can gain only one more electron. neon forms covalent bonds because it has only two electron shells, but bromine has many electron shells and will lose electrons in order to fulfill the octet rule.
Answers: 3

Biology, 22.06.2019 19:30, HtetPaing9281
Analyze how the four requirements for life were identified by scientists
Answers: 1
Do you know the correct answer?
Questions in other subjects:



English, 18.10.2021 04:30


History, 18.10.2021 04:30

Business, 18.10.2021 04:30

Advanced Placement (AP), 18.10.2021 04:30