
Biology, 21.02.2020 18:36, akornegay2
A certain buffer is made by mixing a weak acid HA and its conjugate base A–. When HA is present at a concentration of 0.5 mM and A– is present at a concentration of 0.1 mM, the buffer has a pH of 6.16. Calculate the ratio of the concentrations of HA and A– when the buffer has a pH of 7.02.

Answers: 3
Other questions on the subject: Biology

Biology, 21.06.2019 20:00, hd14yarnell
Use the drop-down menu to match the following definitions to the corresponding terms. the total variety of organisms that live in the biosphere a group of organisms that breed and produce offspring that can breed all of the biotic and abiotic factors in an area
Answers: 1

Biology, 22.06.2019 19:30, madiballet125
You are given an electron micrograph of a bacterial cell. in the micrograph you can clearly see three thin layers of different densities surrounding the cell. based on the micrograph, you can infer that this cell is and would appear after application of the gram stain procedure. gram-negative / pink gram-positive / purple gram-positive / pink gram-negative / purple
Answers: 2

Do you know the correct answer?
A certain buffer is made by mixing a weak acid HA and its conjugate base A–. When HA is present at a...
Questions in other subjects:











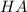 and
and 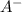 when the buffer has a pH of 7.02 is 0.69
when the buffer has a pH of 7.02 is 0.69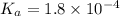
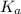 .
.
![pH=pK_a+\log \frac{[A^-}{[HA]}](/tpl/images/0519/3185/d76d0.png)
![6.16=pK_a+\log (\frac{[0.1]}{0.5})](/tpl/images/0519/3185/4ed04.png)
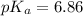
![7.02=6.86+\log \frac{[A^-]}{[HA]}](/tpl/images/0519/3185/a5047.png)
![\frac{[A^-]}{[HA]}=1.44](/tpl/images/0519/3185/0f1f0.png)
![\frac{[HA]}{[A^-]}=0.69](/tpl/images/0519/3185/ef74e.png)




