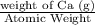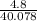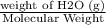4.8g of calcium is added to 3.6g of water. the following reaction occurs ca + 2h2o ca(oh)2 + h2 i. the number of moles of calcium = ii. the number of moles of water = iii. which reagent is in excess? explain your choice. iv. calculate the mass of the reagent named in (ii) which remained at the end of the experiment.

Answers: 1
Other questions on the subject: Biology

Biology, 21.06.2019 14:00, tiffanyhmptn
The population of bacteria in a petri dish doubles every 40 seconds. the population of the bacteria is initially 400 organisms. what unit is most appropriate for the time at which the number of bacteria is 300? hour bacteria per hour second bacteria per second
Answers: 1

Biology, 22.06.2019 00:00, linnybear300
Which ideas did your answer contain? check all that apply. no food for organisms no oxygen in the atmosphere no trees or flowering plants no products based on trees or plants (building materials, medicines, fuels, fibers) no fossil fuels
Answers: 3

Biology, 22.06.2019 02:00, iixyloa
Research cheetahs on the internet what has contributed to this animal becoming "endangered" or "threatened." what animal you have chosen? -cheetah how long has the animal been endangered or threatened? what has contributed to this animal’s endangered or threatened status? why is it important to save this animal from extinction? after researching and gathering facts, write a 350-word letter from the point of view of an animal rights' activist. be sure to include at least five facts that you learned from your research.
Answers: 3

Biology, 22.06.2019 04:00, shayshayyy41
What is the difference between how ionic and covalent bonds form
Answers: 1
Do you know the correct answer?
4.8g of calcium is added to 3.6g of water. the following reaction occurs ca + 2h2o ca(oh)2 + h2 i....
Questions in other subjects:

Biology, 20.09.2020 06:01


Biology, 20.09.2020 06:01



Chemistry, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01

Chemistry, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01