
Advanced Placement (AP), 26.02.2021 16:40, jdvazquez18p7a7vs
For the equilibrium: 2 NO (g) ⇌ N 2(g)+ O 2(g)at 300 K, the equilibrium constant, Kc, is 0.185. If 1.45 moles each of N 2(g)and O 2(g)are introduced in a container that has a volume of 6.00 liters and allowed to reach equilibrium at 300 K, what are the concentrations of N 2(g ) , O 2(g) ,and NO (g)at equilibrium?

Answers: 1
Other questions on the subject: Advanced Placement (AP)

Advanced Placement (AP), 23.06.2019 04:31, kadenbaker4347
Facts/data/information/reason to prove the us is democratic
Answers: 2

Advanced Placement (AP), 25.06.2019 01:30, caitybugking
General ways to reduce the risk of some action include
Answers: 1

Advanced Placement (AP), 25.06.2019 06:00, carlomorales200
Why is it essential to operationally define the variables in a study?
Answers: 1

Advanced Placement (AP), 25.06.2019 22:30, Adeenieweenie
Bytes are commonly used to identify the size of digital information. how many bits are in a byte? 1 2 8 10
Answers: 1
Do you know the correct answer?
For the equilibrium: 2 NO (g) ⇌ N 2(g)+ O 2(g)at 300 K, the equilibrium constant, Kc, is 0.18...
Questions in other subjects:

Mathematics, 31.08.2021 01:50

Mathematics, 31.08.2021 01:50





Physics, 31.08.2021 01:50

Mathematics, 31.08.2021 01:50

History, 31.08.2021 01:50


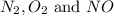 at equilibrium are 0.112 M, 0.112 M and 0.260 M
at equilibrium are 0.112 M, 0.112 M and 0.260 M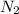 = 1.45 mole
= 1.45 mole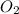 = 1.45 mole
= 1.45 mole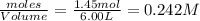

![K_c=\frac{[N_2]\times [O_2]}{[NO]^2}](/tpl/images/1150/8878/68b6f.png)





