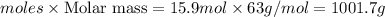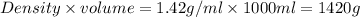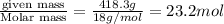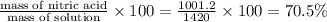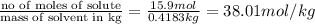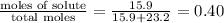Advanced Placement (AP), 06.01.2021 19:50, Lkirjnnfcxd5039
Concentrated nitric acid has a molarity of 15.9 M and a density of 1.42 g/mL. Calculate the following concentrations using 1.00 L of HNO3.
a) % by mass
b) molality
c) mole fraction

Answers: 3
Other questions on the subject: Advanced Placement (AP)

Advanced Placement (AP), 23.06.2019 11:50, yungkxng57
Quickly! ill give you brainliest + free points the best approach to keeping your car in safe working order is to a. have a friend inspect it b. get a professional tune-up c. listen to the engine while it's running d. clean the interior and exterior once a month
Answers: 1

Advanced Placement (AP), 23.06.2019 12:50, gonzalesnik
Want free points + free brainliest? answer this drivers ed question correctly and i got you! a higher grade number for oil means it is a. heavier b. more viscous c. less viscous d. more important
Answers: 2


Advanced Placement (AP), 25.06.2019 01:30, farashka03
Which two organisms in the food web would most likely be affected by a decrease in producers, or the plants, at the bottom base of the food web?
Answers: 1
Do you know the correct answer?
Concentrated nitric acid has a molarity of 15.9 M and a density of 1.42 g/mL. Calculate the followin...
Questions in other subjects:

Mathematics, 13.05.2021 01:50



English, 13.05.2021 01:50

Arts, 13.05.2021 01:50

Mathematics, 13.05.2021 01:50





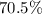
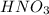 = 15.9 moles in 1.00 L of solution
= 15.9 moles in 1.00 L of solution